The US Food and Drug Administration (FDA) yesterday announced its approval for the use of Pfizer's new respiratory syncytial virus (RSV) vaccine in pregnant women to help protect infants as old as 6 months old, marking a new way to protect babies—a group that is at higher risk for complications from the virus.
The US Food and Drug Administration (FDA) yesterday announced its approval for the use of Pfizer's new respiratory syncytial virus (RSV) vaccine in pregnant women to help protect infants as old as 6 months old, marking a new way to protect babies—a group that is at higher risk for complications from the virus.
FDA's approval is for use of the vaccine at 32 to 36 weeks gestational age of pregnancy. The vaccine, called Abrysvo, is administered as a single intramuscular dose. Pregnant women in this latter stage of pregnancy are the second group approved to receive Abrysvo; in May, the FDA cleared the vaccine for use in adults ages 60 and older.
Yesterday's approval announcement follows recommendations earlier in May from the FDA's vaccine advisory group, which unanimously approved a vote on efficacy, but raised some concerns about preterm births in a safety vote that passed by a tighter margin. The prescribing information includes information about a numerical imbalance in preterm births among Abrysvo recipients (5.7%) compared to those who received placebo (4.7%). The FDA, however, said the data aren't sufficient to establish or exclude a relationship between Abrysvo and preterm birth. Also, the warning urged healthcare providers to avoid using the vaccine in women before 32 weeks gestation.
CDC next to weigh in
Peter Marks, MD, who directs the FDA's Center for Biologics Evaluation and Research, said in the statement that infants are at risk for severe disease, which can lead to hospitalization. "This approval provides an option for healthcare providers and pregnant individuals to protect infants from this potentially life-threatening disease."
Before vaccination begins, the Centers for Disease Control and Prevention (CDC) vaccine advisory group will discuss a recommendation, which will require a final sign-off by the CDC. Earlier this month, the CDC recommended the nirsevimab shot, a long-acting monoclonal antibody developed by AstraZeneca, to protect infants younger than 8 months old against RSV.
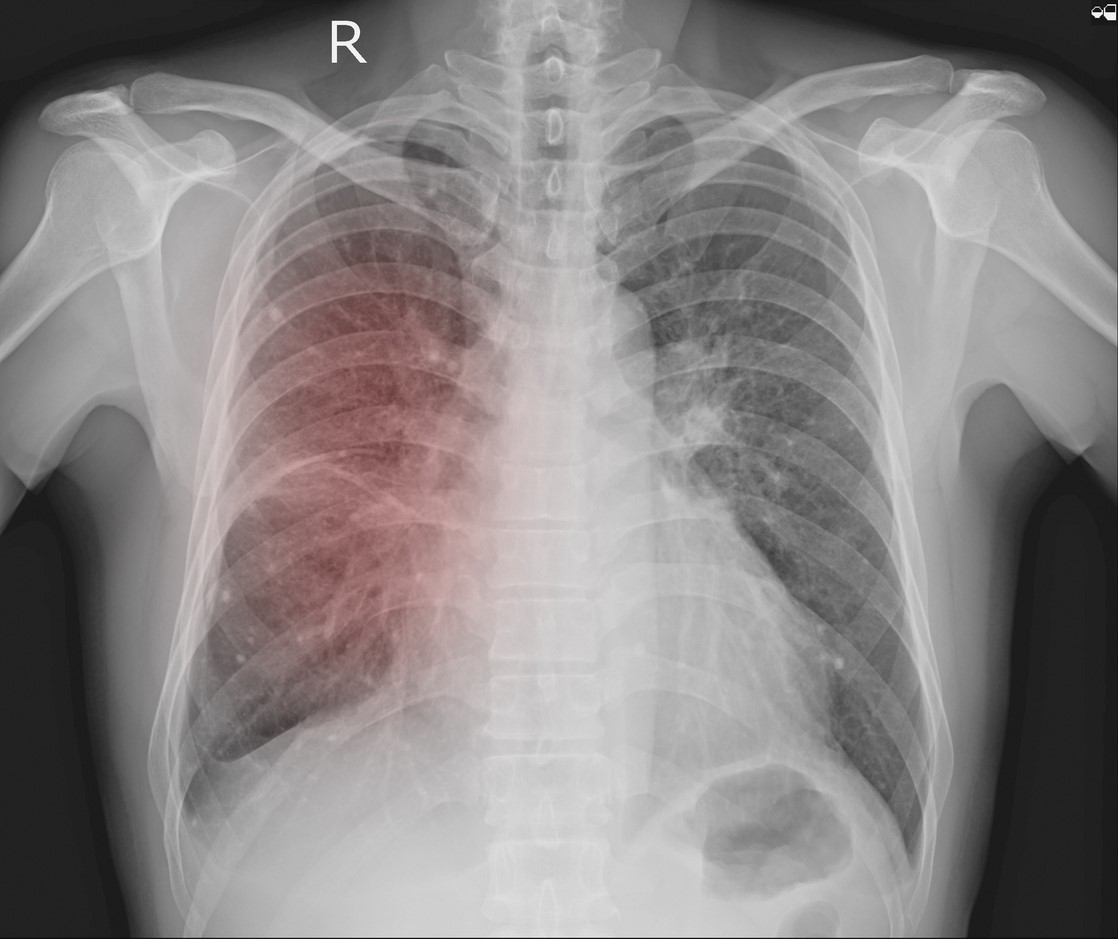 University of Chicago researchers report that they have built a deep-learning (artificial intelligence [AI]) model that can evaluate chest x-rays (CXRs) to predict whether COVID-19 patients will need intensive care, aiding triage and hospital resource allocation during patient surges.
University of Chicago researchers report that they have built a deep-learning (artificial intelligence [AI]) model that can evaluate chest x-rays (CXRs) to predict whether COVID-19 patients will need intensive care, aiding triage and hospital resource allocation during patient surges.