Outgoing Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, MPH, yesterday endorsed last week's Advisory Committee on Immunization Practices (ACIP) recommendation on use of two newly approved respiratory syncytial virus (RSV) vaccines in people ages 60 and older. The recommendation stipulates that people can get a single dose of the vaccine based on shared clinical decision making, meaning it isn't recommended for everyone in the age group.
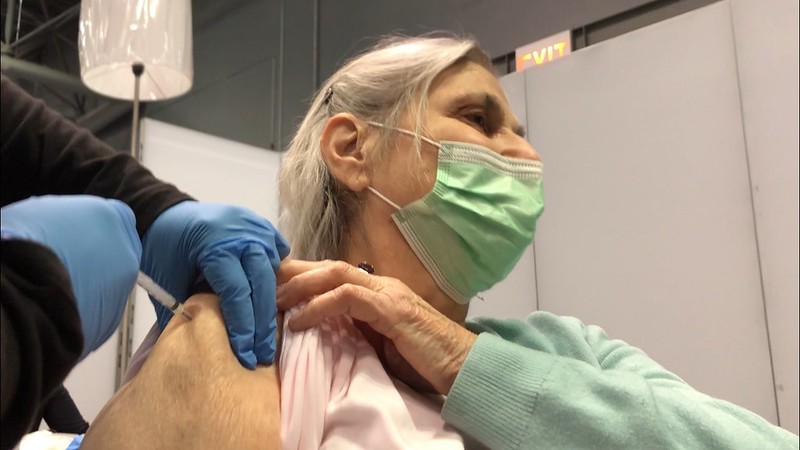
The CDC said ACIP makes a shared clinical decision-making recommendation when individuals may benefit from vaccination, but broad vaccination isn't likely to have population-level impacts. Examples of other vaccines that have a shared clinical decision-making recommendation component include the meningococcal B vaccine for adolescents and young adults and the hepatitis B vaccine for adults age 60 and older who have diabetes. For such a recommendation, the CDC defines health provider as anyone who administers vaccines, including primary care physicians, specialists, physician assistants, nurse practitioners, registered nurses, and pharmacists.
The CDC said the vaccines are expected to be available this fall and that immunization provides an opportunity to protect older adults against severe RSV illness at a time when multiple respiratory viruses are expected to be circulating.
.jpg) An
An 









