Foster Farms recalls chicken linked to 621-case Salmonella outbreak
As the United States celebrated Independence Day late last week, federal officials announced that Foster Farms has recalled chicken products implicated in a Salmonella outbreak that has now reached 621 cases in 29 states.
In a late–Jul 3 notice that was reissued on Jul 4 with updated information, the US Department of Agriculture's Food Safety and Inspection Service (FSIS) said Foster Farms, of Livingston, Calif., has recalled chicken products produced in early March after the company's chicken was definitively linked to a patient in the outbreak.
This is the first direct evidence tying a Foster Farms product to an outbreak patient, though federal officials have long linked the outbreak to the company's products.
Direct evidence is not easy to obtain, the FSIS pointed out, as it entails obtaining product from a case-patient that tests positive for the outbreak Salmonella strain, plus packaging must clearly associate the item to a specific facility and production date. Also, officials must obtain documentation of shipping and distribution to trace the product to the facility.
The recall involves chicken produced from Mar 7 through Mar 13, the FSIS said. The products total more than 1 million pounds, Food Safety News (FSN) reported today.
"These products were shipped to Costco, Foodmaxx, Kroger, Safeway and other retail stores and distribution centers in Alaska, Arizona, California, Hawaii, Idaho, Kansas, Nevada, Oklahoma, Oregon, Utah, and Washington," the FSIS notice said.
Jul 4 FSIS recall notice
Jul 7 FSN report
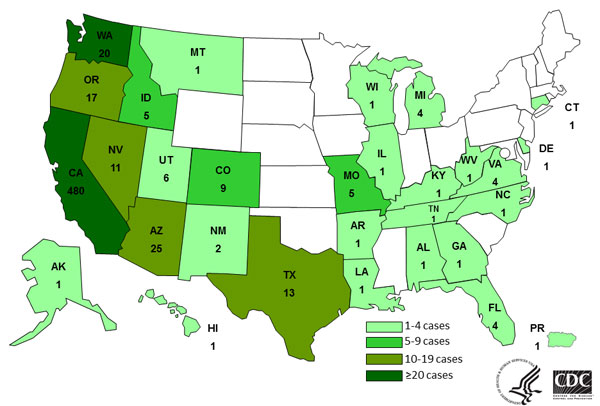
In related news, the Centers for Disease Control and Prevention (CDC) reported on Jul 4 that the outbreak now totals 621 cases, up from 574 cases in the CDC's last update on May 27.
The 47 new cases are from five states: California, 39; Oregon, 3; Washington, 3; Alabama, 1; and West Virginia, 1. Of the 621 outbreak cases, 480 (77%) have been in California (see CDC map below).
Illness-onset dates in the outbreak, which is caused by a Salmonella Heidelberg strain, range from Mar 1 to Jun 15. Patient ages range from less than 1 year to 93 years, with a median of 18 years. Of 504 patients with available information, 181 (36%) were hospitalized.
Blood infections have developed in 13% of outbreak patients, compared with 5% in a typical Salmonella outbreak. There have been no deaths reported.
Jul 4 CDC update
May 27 CIDRAP News scan on previous CDC update
CDC fine-tunes polio vaccine guidance for travelers
The CDC today issued interim polio vaccination guidance for US travelers as it formalized advice it gave last month in a Health Alert Network (HAN) advisory, highlighting possible requirements for those traveling to polio-infected countries.
The CDC notes in a Morbidity and Mortality Weekly Report (MMWR) early release that the World Health Organization (WHO) has now listed four nations as "exporting wild poliovirus"—Cameroon, Equatorial Guinea, Pakistan, and Syria. Equatorial Guinea was not on that list when the Jun 2 HAN notice was posted. Those countries should "ensure" recent polio boosters among departing residents and those visiting the countries for more than 4 weeks, says the guidance.
The other six polio-infected nations—Afghanistan, Ethiopia, Iraq, Israel, Nigeria, and Somalia—should "encourage" the boosters. The CDC reminds clinicians, "All polio vaccination administration should be documented on an International Certificate of Vaccination or Prophylaxis (often referred to as the WHO 'yellow card')."
The MMWR article also details general recommendations for polio vaccination according to guidelines established in 2009 by the CDC's Advisory Committee on Immunization Practices.
Jul 7 MMWR article
Jun 3 CIDRAP News item on HAN alert











