About a year ago—on Nov 11, 2015—Brazil declared a national emergency after 141 babies were born with microcephaly, or abnormally small heads and brains. The mothers of these babies had been infected with Zika virus, a mosquito-borne flavivirus that usually causes mild to not symptoms, in pregnancy, and officials strongly suspected a link.
Now, as Zika continues to march across large swaths of the globe and thousands more babies have been born with congenital disorders, the race for a Zika vaccine is at full throttle. And experts in the field say that it's likely there will one day be at least two vaccines used to beat back the virus.
"We have at least five vaccines with our fingerprint on them," said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID). "Some further along than others."
According to Fauci, this is one of the most hotly pursued vaccines in medical history, with dozens of companies, government agencies, and nations throwing billions of dollars into research and development (a BMJ editorial last week said there were about 30 Zika vaccine projects in the works).
Because of the unique properties of the disease, including the strong possibility it will become endemic in parts of South America, a vaccine is necessary to prevent microcephaly and other birth defects.
"Zika could be something like rubella, where the only way to prevent birth defects is to vaccinate everyone in childhood and protect future pregnancies," said Fauci.
But unlike rubella, Zika will likely be combatted by two, and not one, vaccines: First a DNA-based vaccine that will confer immunity for a shorter period and could help stamp out the current outbreak, and later a live-attenuated-virus–based vaccine that could offer lifelong immunity to recipients.
DNA-based for outbreaks, travelers
While Fauci said it's impossible to tell which vaccine will most likely be the first to succeed and begin protect people from the mosquito-borne illness, he said a few are more "temporally likely" than others.
"A straightforward DNA-based vaccine could be used in the middle of an outbreak, or before travel to an area experiencing an outbreak," said Fauci. In that way, the Zika vaccine would act like dengue or yellow fever immunization. In fact, because Zika is a flavivirus, and so many flaviviruses have successful vaccines, researchers and pharmaceutical companies have been quick to assume Zika will be similar.
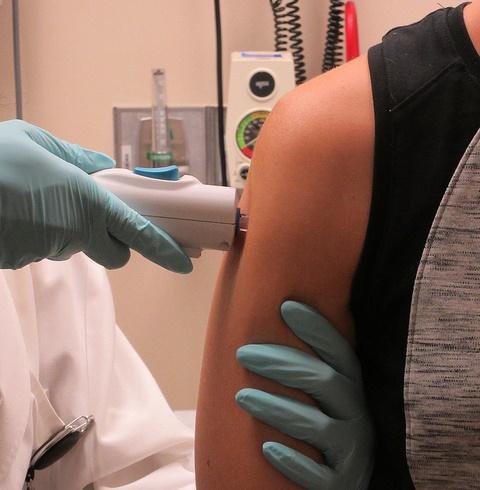
In August, NIAID's Vaccine Research Center began human trials of its investigational Zika vaccine (VRC 319), which includes a small, circular piece of DNA—called a plasmid—that contains genes that code for proteins of the Zika virus. When injected into muscle, the plasmid helps the body make virus-like particles.
"The speed with this vaccine [development] is unprecedented," said Fauci. Currently VRC 319 is being tested in humans in phase 1 trials at three clinical sites.
That speed is due to a number of factors, said Sri Edupuganti, MD, MPH. Edupuganti is heading the clinical trial of VRC 319 at Emory University. She and her colleagues have tested the safety of and immune response to the injection in 11 adults, with plans to increase the study size to 30 in the coming weeks.
"Because of the last 20 years of vaccine development, mainly because of HIV vaccine work, we've benefited from technology and strategies to quickly work on a Zika vaccine," said Edupuganti. She explained that VRC 319 is also based on the now-abandoned West Nile virus vaccine (no major pharmaceutical companies invested in that vaccine).
"Using West Nile as a backbone allowed us to be very nimble," said Edupuganti.
Live-attenuated vaccine for endemic regions
While some DNA vaccines are already in clinical trials, a research group at the University of Texas Medical Branch (UTMB) in Galveston was one of the first approached by the Brazilian Ministry of Health in February to begin work on a live-attenuated-virus vaccine.
"When you look at the most successful vaccines, polio, yellow fever, Japanese encephalitis, these are all live-attenuated vaccines," said Pei-Yong Shi, PhD, a molecular biologist at UTMB. Shi's group is using a unique reverse-genetic system to craft its vaccine. It's the first time such technology will be used in vaccine development, and Shi said it allows his group to make a live-attenuated vaccine that's safe for recipients.
"We can manipulate the virus anywhere in its genome like a Lego block. We take out the disease-causing elements, weaken the virus, and produce an effective and safe vaccine," said Shi.
He said a live-attenuated virus-based vaccine is the best long-term solution for a disease with far-reaching consequences for countries like Brazil. Because DNA-based vaccines may need boosters or may confer immunity for only a short period, developing a safe vaccine that can be given in childhood and offer lifelong immunity before recipients bear children is the best way to protect fetuses from Zika.
"Even in an endemic situation you would still have outbreaks, so there needs to be a safe and long-lasting vaccine," said Shi, who said his team is currently working with the Instituto Evandro Chagas and National Reference Laboratory for Arbovirus in Brazil. "So there's probably a role for more than two types of vaccine."
Shi would not say when the live-attenuated vaccine would be ready for trials. Unlike the DNA-based vaccine, a live-attenuated vaccine will have a higher safety hurdle to jump because it contains parts of the Zika virus.
More interest, more money
Before Zika exploded, the last disease to trigger a substantial wave of vaccine activity was Ebola. But unlike the Ebola virus outbreak of 2014-2016, which prompted the NIAID to fund only three vaccine candidates, the Zika virus has been drawing more interest from major pharmaceutical companies and governments.
"I don't know exactly why there's been so much interest in Zika," said Fauci. "It's probably that Ebola is always an outbreak, while Zika has the possibility of becoming endemic in South America, Africa, and Southeast Asia."
The possibility of Zika becoming endemic in several highly populated countries could mean a profit for companies that produce a Zika vaccine.
See also:
Nov 10 BMJ editorial
Aug 3 Emory University press release on phase 1 trials
Feb 16 UTMB press release on collaboration with Brazil
NIAID Zika vaccine page