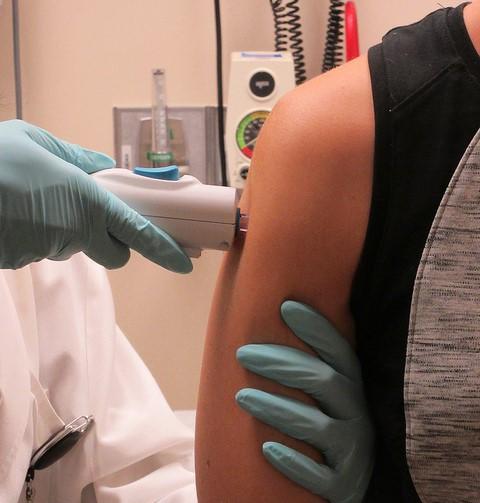
The first results from a human trial of the DNA-based Zika vaccine GLS-5700 showed it was safe and elicited a robust immune response in study participants. Results from the phase 1 trial were published today in the New England Journal of Medicine.
GLS-5700 contains synthetic DNA, which mounts an immune response against specific Zika virus antigens. Pablo Tebas, MD, the lead study author and a researcher at Perelman School of Medicine at the University of Pennsylvania, said the results of the trial are promising.
"We saw neutralizing antibodies mount in participants after 14 weeks," Tebas said in an interview. "And with DNA-based vaccines, it only took 7 months to develop and manufacture this vaccine for the trial. So we could have a Zika vaccine very quickly with this method."
To conduct the study, Tebas and colleagues recruited 40 adults in the summer of 2016 to receive either 1- or 2-milligram (mg) doses of the vaccine intradermally at 0, 4, and 12 weeks. Accompanying each shot was treatment with Cellectra, a device that uses pulsed electric fields to introduce the DNA sequence into cells.
By week 14, 2 weeks after the final dose, all study participants developed Zika-specific antibodies, and 80% had developed neutralizing antibodies against the virus. Any differences in antibody development between the group receiving 1 mg verses 2 mg of the vaccine became statistically insignificant after week 6 of the study.
Serum collected from the participants was then injected into mice, which were then challenged with Zika virus. The serum prevented both infection with Zika virus and death in the animals.
Efficacy will be tested in larger trials
The study did not evaluate the efficacy of the vaccine in preventing human infection with Zika, which will need to be evaluated in an endemic region with a larger study population.
"But the results in our mouse models were very promising," said Tebas, who also noted that there were no serious side effects observed among human participants. About half the participants reported localized redness and irritation at the injection site.
GLS-5700 was developed and tested through a partnership among Inovio Pharmaceuticals, the Wistar Institute, GeneOne Life Science, and the University of Pennsylvania.
"Synthetic DNA vaccines are an ideal approach for emerging infectious diseases like Zika," said David B. Weiner, PhD, executive vice president of the Wistar Institute, in a Wistar press release today. Weiner said that DNA-based vaccines are more stable, predictable, and practical in an emerging outbreak scenario.
The Food & Drug Administration approved GLS-5700 for human clinical trials in June 2016, the first Zika vaccine approved for testing in human volunteers.
See also:
Oct 4 N Engl J Med study
Oct 4 Wistar Institute news release