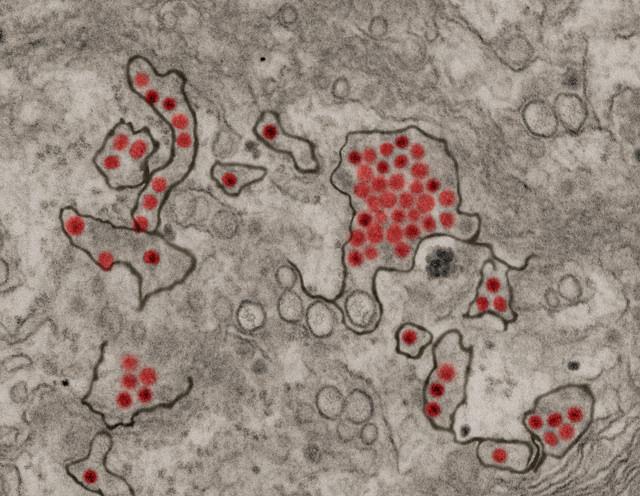
In the latest Zika virus developments, the second local case of the year has been reported in Texas, and the US Food and Drug Administration (FDA) announced that it approved the first test for screening blood donations for the virus.
The case in Texas is from a county where illnesses were reported last year, and the Zika test has already removed hundreds of potentially infected donations from the blood supply, officials said.
Texas case reported in earlier hot spot
The latest local case from Texas involves a woman from Cameron County who likely contracted the virus months ago, according to a statement yesterday from the Cameron County Department of Health and Human Services (CCDHHS).
The patient is a Laguna Heights resident who sought Zika testing at the county's public health clinic. James Castillo, MD, with the Cameron County Health Authority, said "Clinical test results show that this person may have had the virus 2-3 months ago and it is no longer active in her system." He added that there is no evidence of any other related mosquito-transmitted cases.
As a precaution, the CCDHHS has conducted an epidemiologic investigation of the patient's household and shared information with people in neighboring homes on how to eliminate mosquito breeding sources.
Cameron is one of nine Texas counties at the southern tip of the state on the Mexico border where state health officials earlier this year expanded Zika testing recommendations to include all pregnant women and people with Zika symptoms. State health warned of increased mosquito activity heading into the warmer months and of the possibility of Zika reappearing, given the proximity to Mexico, where Zika activity continues.
Cases of dengue have been reported in the Rio Grande Valley before, and as health officials feared, Zika followed its footsteps last summer.
So far, Texas is the only state this year to report recent local Zika cases. Its first illness—announced in late July—involved a resident of Hildago County. Health officials said the individual was probably infected in South Texas in the previous few months and probably represented an isolated case.
Zika test for blood donations
In an announcement yesterday, the FDA said it approved the cobas Zika test, a nucleic acid test that detects Zika RNA in individual plasma specimens from donated whole blood or blood components and from living organ donors.
The agency said the test is intended for blood collection establishments, not for the individual diagnosis of Zika infection.
Peter Marks, MD, PhD, director of the FDA's Center for Biologics Evaluation and Research, said in the statement, "Screening blood donations for the Zika virus is critical to preventing infected donations from entering the U.S. blood supply." He added that the approval signals the manufacturer's commitment to work with the FDA and blood collection industry to respond quickly to the public health threat to ensure the safety of the blood supply of the United States and its territories.
Several blood collection facilities have already been using the cobas Zika test on an investigational new drug basis, based on the FDA's 2016 guidance. The tests is intended for use on fully automated cobas 6800 and 8800 systems, which are made by Roche Molecular Systems, Inc.
Roche said yesterday that the test has screened more than 4 million blood donations from the United States and Puerto Rico and has helped identify and remove more than 450 potentially infectious donations from the blood supply.
See also:
Oct 5 CCDHHS statement
Aug 24 Texas Department of State Health Services statement on expanded Zika testing
Jul 26 CIDRAP News story "Texas reports first local Zika case of 2017"
Oct 5 FDA press release
Oct 5 Roche press release