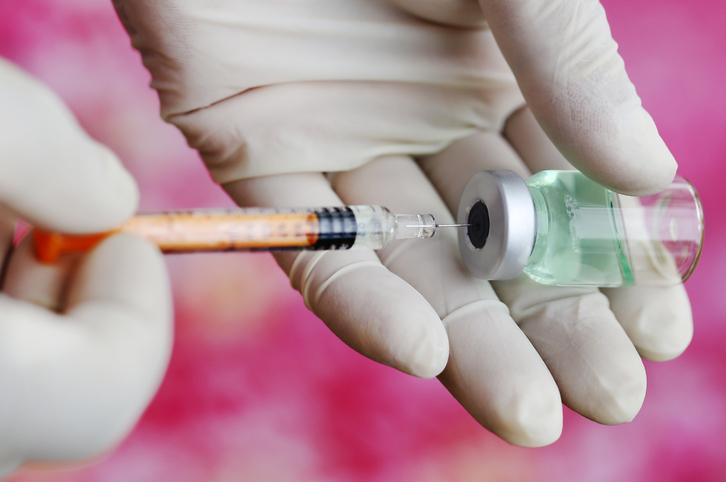
In Zika vaccine developments, two different live-attenuated vaccine candidates progressed, with one research group reporting promising findings in mice and the other announcing that the first trial has been launched in humans.
Also, a study on breastfeeding mother and babies didn't have enough information to say if the virus can be passed through that route.
Vaccine fully protects mice
The animal study evaluated a live-attenuated vaccine containing a deletion in the Zika virus genome (10-del ZIKV), an approach that has been used to develop a dengue vaccine that is now in phase 3 trials. Researchers from the University of Texas Medical Branch at Galveston (UTMB) and the Evandro Chagas Institute in Brazil reported their preclinical trial findings yesterday in Nature Medicine.
Pei-Yong Shi, PhD, senior author and professor in UTMB's department of biochemistry and molecular biology, said in a UTMB press release, "Such live-attenuated vaccine has the advantage of single-dose immunization, rapid and strong immune response, and potentially long-lived protection." He added that a balance is needed with this type of vaccine between fast, durable immunity and safety.
Inactivated vaccines may be safer but may initially require several doses and periodic boosters. "Therefore, a safe live-attenuated vaccine will be ideal in prevention of Zika virus infection, especially in developing countries," he said. The initial target of the vaccine is childbearing-age women, their sexual partners, and children younger than 10 years old.
Public health experts have said multiple Zika platforms will probably be needed, such as a DNA-based product for outbreaks or use before travel and a live-attenuated vaccine for protecting people in areas where Zika is endemic. Attenuated vaccines are typically contraindicated in people with weakened immune systems, and health officials are eying DNA-based vaccines as a safer option for use in pregnant women.
In mice, a single dose prompted sterilizing immunity in mice, preventing all Zika infection. The team said sterilizing immunity is crucial for preventing viremia and birth defects.
When researchers looked at viral loads in different mouse organs, they found low and transient viral loads. They also observed limited weight loss and lack of deaths, suggestive of a promising safety profile.
They concluded that the findings support advancing the study of the vaccine to nonhuman primate trials.
Human trial launch
Meanwhile, Themis Bioscience, a biotech company based in Austria, announced today the launch of a live-attenuated recombinant Zika vaccine in humans. The vaccine, called MV-ZIKA-101, is based on vaccine vector technology that uses a measles virus as its backbone. Themis said in a statement today that it had earlier used the same technology to develop a candidate chikungunya vaccine.
The goal of the phase 1 trial is to identify the most suitable dose regarding immunogenicity, safety, and tolerability. One group will receive one injection of high-dose vaccine, and the other two will receive two doses of either high-dose or low-dose vaccine. Up to 48 healthy volunteers will be enrolled in Vienna, comparing the three regimens with a placebo.
Themis said the trial is the first to test a live-attenuated vaccine in humans and that the measles vaccine model has a proven efficacy and safety track record over the last 30 to 40 years. Results are expected in the next 6 months.
Breastfeeding questions
In other Zika developments, a case review of breastfeeding women with symptomatic Zika virus infection within 3 days of delivery didn't have enough data to conclude that Zika transmission occurred through that body fluid, researchers reported yesterday in PLoS Neglected Tropical Diseases.
Researchers from Cornell University in New York and the World Health Organization based their review on two medical literature reports that included three mother-child pairs: two in a case report from French Polynesia's Zika outbreak and the other a case report on a pair from New Caledonia.
In two of the three cases, newborns had evidence of Zika infection. Zika virus was detected in breast milk of all three mothers, one of which was positive by culture.
Researchers said despite the breast milk findings, the data aren't sufficient to confirm Zika transmission through breastfeeding and that more evidence is needed to distinguish transmission through that route from other perinatal transmission possibilities.
Earlier this year, a team from Brazil reported on four Zika-infected breastfeeding mothers from that country, finding no illnesses in the babies except for fever and rash in one that was related to chikungunya infection. One of the mothers' breast milk samples yielded infectious virus.
That group said Zika transmission by breast milk is questionable and might be less efficient for transmitting the virus than other body fluids.
See also:
Apr 10 Nat Med abstract
Apr 10 UTMB press release
Apr 11 Themis press release
Apr 10 PLoS Negl Trop Dis study
Feb 9 CIDRAP News story "Studies highlight microcephaly data, Zika spread by breast milk, saliva"