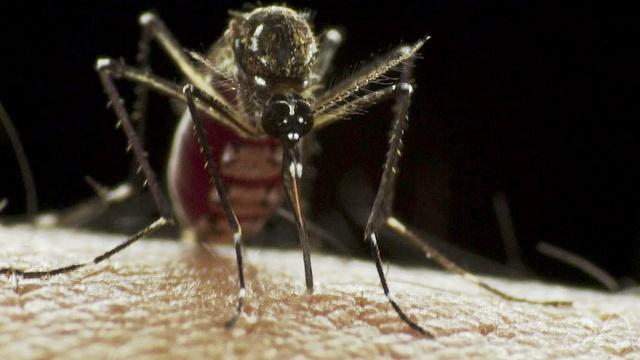
As part of the ramp-up in response to Zika virus outbreaks, a 2-day emergency meeting of the World Health Organization's (WHO's) vector control advisory group began in Geneva today with the goal of discussing new tools, given a recent assessment that current tools are having little impact against the Aedes mosquitoes that spread the virus.
Last week at a meeting in Geneva to set research and development priorities on tools to battle the disease in humans, such as vaccines and diagnostic tests, mosquito experts described how traditional approaches such as spraying haven't been able to interrupt dengue transmission, raising concerns that they wouldn't help much in the battle against Zika virus, either, as both pathogens are spread by A aegypti mosquitoes.
Feasibility of new mosquito tools
In a statement on the meeting, the WHO said the expert group will review existing mosquito control methods as well as the evidence on proposed vector control tools, with an eye toward identifying gaps that might limit their use.
The new tools under discussion include Wolbachia bacteria, transgenic mosquitoes (Oxitec OX513A), sterile insect technique (SIT), vector traps, and attractive toxic sugar baits. At the meeting's conclusion, the group will issue a position statement on existing and new vector control tools for use in response to Zika virus outbreaks.
Brazil's National Biosafety Committee has already approved the release of Oxitec's "self-limiting" mosquitoes, and Oxtitec projects are under way in a few Brazilian locations and have shown promising results, according to an earlier report.
Last week the International Atomic Energy Agency's governing board approved $2.5 million to help Latin American and Caribbean countries acquire the technology to use SIT to reduce mosquito populations. The technique uses gamma cell irradiation to sterilize male mosquitoes.
FDA eyes modified mosquitoes
In a related vector-control development, the US Food and Drug Administration (FDA) on Mar 11 released a draft environmental assessment from Oxitec on the potential environmental impact of a field trial of genetically modified A aegypti mosquitoes slated to take place in the Florida Keys. The FDA also released its preliminary findings of no significant impact from the field trial, which agrees with Oxitec's draft report.
The FDA said that, based on the findings of a review team, toxic or allergenic effects of exposure to the proteins in the OX513A mosquitoes would likely be negligible, since the mosquitoes released will be male, and male mosquitoes don't bite humans or animals. "They are therefore not expected to have any direct impacts on human or animal health."
The agency also added that the release would be highly unlikely to contribute to an increase in transmission of dengue or other mosquito-borne diseases.
The trial is slated for Key Haven, a suburb of Key West that is home to about 1,000 people.
Oxitec won't conduct a field trial of its OX513A mosquito until the FDA has reviewed public comments on the draft environmental assessment and issued a final impact statement. The FDA is accepting public comments on the two reports for 30 days from the publication of its Mar 14 Federal Register notice. The FDA encourages the public to submit written or electronic comments by Apr 13.
Protecting workers, risk communication, GBS in Honduras
- The WHO recently released initial guidance on protecting workers involved with A aegypti mosquito control efforts. It covers occupational safety measures and how to manage acute insecticide poisoning.
- To help countries plan their Zika public messaging campaigns, the WHO and its partners recently released Zika prevention and control risk communication and community engagement guidance. The 17-page guide is available in both English and Spanish, covering topics such as core messages and community-based behaviors for eliminating mosquito breeding sites and protective behaviors, especially for high-risk groups. WHO collaborators included its Pan American Health Organization office, the United Nations Children's Fund (UNICEF), and the International Federation of Red Cross and Red Crescent Societies.
- Honduran health officials reported a death from Zika-related Guillain Barre syndrome (GBS), Agence France-Presse (AFP) reported on Mar 11, citing the country's deputy health minister. About nine Zika-hit countries or territories have reported rises in GBS cases, though reports of deaths have been few. The health minister said the country has received 57 reports of GBS potentially linked to recent Zika virus infection.
See also:
Mar 14 WHO statement on emergency vector control meeting
Mar 11 FDA statement
Mar 14 Federal Register notice