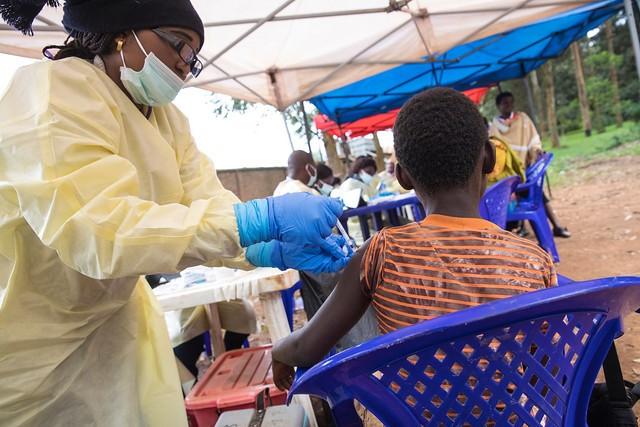
According to the latest weekly bulletin from the World Health Organization's (WHO's) African regional office, the Ebola outbreak in the Democratic Republic of the Congo (DRC) is experiencing a drop in the rate of new cases, but it's too early to say if that drop is a significant trend forecasting the end of the 13-month-long outbreak in the DRC's North Kivu, South Kivu, and Ituri provinces.
"There are early signs of the outbreak easing, including the decrease in numbers of new confirmed cases in Beni and Mandima and the lack of new confirmed cases for 21 days in Alimbongo and Oicha," the WHO said. "However, it is too soon to know if this is an indication of a decrease in transmission intensity of [Ebola virus disease]."
In the past 21 days, 13 health zones confirmed cases of Ebola, and officials confirmed 37 new cases since Sep 8. In September, the virus hot spots have been Kalunguta (22% of new cases) Mambasa (17%), Manama (15%), and Beni (13%).
"A total of 13,294 contacts are under follow-up as of 13 September 2019, of which 11,891 have been seen in the past 24 hours, comprising 89% of the contacts, comparable to that during the past seven days (90%)," the WHO said. As of Sep 13, 17% of all alerts concerning Ebola were deemed suspected cases.
As of today, the WHO's Ebola dashboard is showing just 1 newly confirmed case, raising the outbreak total to 3,130, including 2,096 deaths. A total of 402 suspected cases are still under investigation.
Today the DRC's multisector Ebola response committee (CMRE) offered new details on the four cases reported yesterday. Two were in Kalunguta, with one each was in Mambasa and Mandima.
FDA priority review for Merck vaccine
In other news today, the US Food and Drug Administration (FDA) has granted priority review for Merck's investigational Ebola vaccine (V920, also known as VSV-EBOV) for preventing disease caused by the Ebola Zaire virus, Merck said in a news release.
"Merck has worked with government partners and the global health community to accelerate development of our investigational V920 Ebola vaccine. FDA’s priority review designation underscores our long-standing partnership with the U.S. government toward its development and licensure," said Paula Annunziato, MD, vice president of Merck Research Laboratories.
"A top priority for us remains achieving registration of V920 and regulatory approval of our German manufacturing site, so that licensed supply can be produced over time to support global public health preparedness and health security objectives. We look forward to continuing to work with the FDA throughout the review process."
The vaccine is currently being used in a ring vaccination campaign throughout the outbreak region. As of Sep 13, a total of 220,529 people have been vaccinated in the past year with the vaccine, which was first tested in 2014, during the West African Ebola outbreak.
The vaccine has proved safe and efficacious on the frontlines of the current outbreak, with recent studies published this spring showing an estimated vaccine efficacy rate of 97.5%.
According to Merck, since May 2018, the company has given the WHO 245,000 1-milliliter investigational doses of the vaccine and has another 190,000 doses available for shipment.
Former DRC health minister arrested
Yesterday Science magazine reported that Congolese police arrested Oly Ilunga Kalenga, MD, the former health minister of the DRC, on charges of mismanaging $4.3 million in Ebola response money.
Kalenga oversaw the outbreak response for the first 11 months of the outbreak, before resigning from his post in July. At that time, the DRC’s president, Felix Tshisekedi, created the CMRE to handle outbreak response.
Kalenga was arrested in Kinshasa, where he was said to have been planning an escape from the DRC. His lawyers have denied the allegations, calling them slanderous.
See also
Sep 15 WHO African Regional Office update
WHO Ebola dashboard
Sep 17 Merck press release
Apr 15 CIDRAP News story “Ebola cases climb by 44 as vaccine trial affirms high efficacy”
Sep 16 Science story