The Florida Department of Health (DOH) this week issued a mosquito warning after officials confirmed two cases of locally acquired malaria in the state, the first in 20 years.
People in Sarasota and Manatee counties contracted the mosquito-borne infection in late May. One of the patients has been treated and released, and the other is still being treated, the DOH said in a news release.
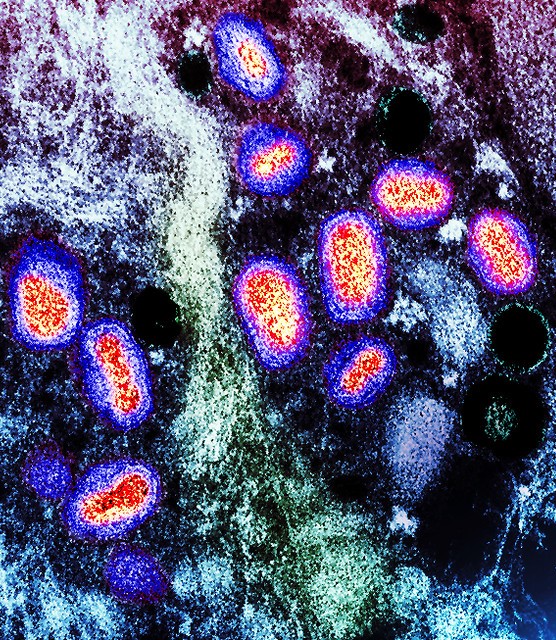
Officials confirmed that the cases are caused by the Plasmodium vivax malaria parasite, which is not as fatal as other malaria species. "Only infected Anopheles mosquitoes can transmit malaria to humans," the DOH said. It added, "Individuals in this area with symptoms of fever, chills, sweats, nausea/vomiting, and headache should seek immediate medical attention."
Individuals in this area with symptoms of fever, chills, sweats, nausea/vomiting, and headache should seek immediate medical attention.
The DOH said residents in the affected counties should take precautions, such as wearing long-sleeved shirts and pants, applying mosquito repellent, and avoiding areas with high mosquito populations, especially during sunrise and sunset, when mosquitos are most active. The agency also advised people to drain standing water to limit mosquito breeding grounds. A previous DOH release noted that response efforts include aerial and ground spraying to control mosquitoes in the affected counties.
These are the first locally acquired malaria cases confirmed in Florida since 2003, the Mirror reported today.