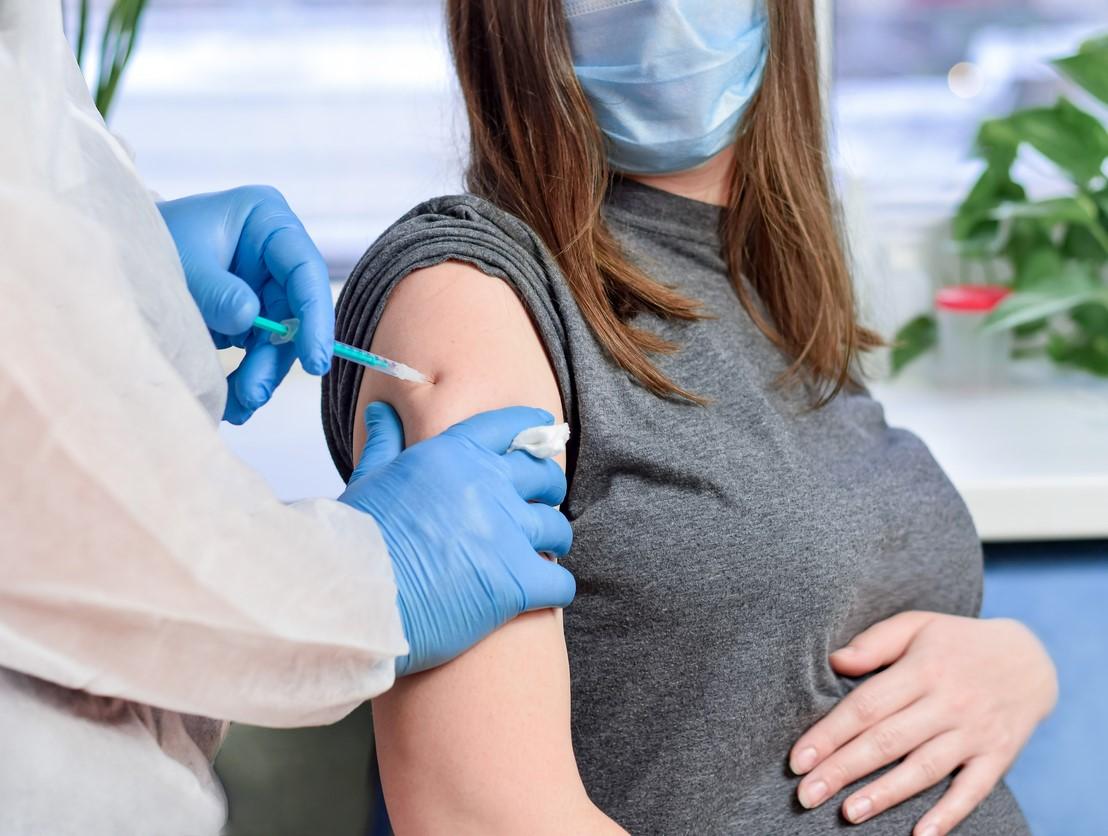
COVID-19 vaccination can prompt a vigorous immune response in pregnant women, who then pass the resulting antibodies on to their newborns through the placenta and breast milk, according to a study published yesterday in the American Journal of Obstetrics and Gynecology.
Led by researchers at Brigham and Women's Hospital and Massachusetts General Hospital, who call it the largest such study yet, the prospective analysis involved the blood and breast milk of 131 women of reproductive age, including 84 pregnant, 31 lactating, and 16 nonpregnant participants, who received two doses of either the Pfizer/BioNTech or Moderna mRNA COVID-19 vaccine from Dec 17, 2020, to Mar 2, 2021.
Blood and breast milk samples were collected at the time of the women's first and second vaccine doses, 2 to 6 weeks after the second dose, and at delivery (for the 13 pregnant women who gave birth during the study period). The researchers also collected umbilical cord blood at delivery. Five women reported previous infection with SARS-CoV-2, the virus that causes COVID-19.
Transfer of antibodies from moms to newborns
The investigators found that the antibody levels induced by the vaccine were uniformly high in all three groups of women. "COVID-19 vaccination in pregnancy and lactation generated robust humoral immunity similar to that observed in non-pregnant women with similar side effect profiles," the authors wrote.
"While humoral [antibody-mediated] immune response and side effects are only two of many considerations for pregnant women and their care providers in weighing whether or not to be vaccinated against COVID-19 in pregnancy, these data confirm that the COVID-19 mRNA vaccines result in comparable humoral immune responses in pregnant and lactating women to those observed in non-pregnant populations," they said.
Side effects were rare and occurred with similar frequency in all participants, including fever and chills (32% of pregnant women after the first vaccine dose, 50% of nonpregnant women).
Vaccine-induced antibodies were found in all umbilical cord blood and breast milk samples, indicating the transfer of antibodies from mothers to infants. Although antibody levels were higher in the mothers' blood than in umbilical cord blood, the difference was not statistically significant. And compared with antibody levels induced by natural COVID-19 infection in pregnancy, vaccination-induced antibody levels were significantly higher.
The second dose of COVID-19 vaccine increased concentrations of coronavirus-specific immunoglobulin G (IgG) but not immunoglobulin A (IgA). And levels of mucosal (IgA) antibodies generated by the second dose of the Moderna vaccine were higher than those induced by two doses of the Pfizer version.
"This finding is important for all individuals, since SARS-CoV-2 is acquired through mucosal surfaces like the nose, mouth and eyes," study first author Kathryn Gray, MD, PhD, said in a Massachusetts General Hospital news release. "But it also holds special importance for pregnant and lactating women because IgA is a key antibody present in breastmilk."
Among pregnant participants, mean gestational age at first vaccine dose was 23.2 weeks. Eleven (13%) received their first dose in the first trimester, 46% in the second trimester, and 40% in the third trimester.
Inclusion of pregnant women in vaccine trials
The authors noted that pregnant women can experience more severe COVID-19 infection than their nonpregnant peers, including higher risk of hospitalization, intensive care unit stay, and death.
Thus, the finding of robust immune response is encouraging for pregnant and breastfeeding women, who weren't included in the initial COVID-19 vaccine trials, study co-senior author Andrea Edlow, MD, MSc, said in the release. "Filling in the information gaps with real data is key—especially for our pregnant patients who are at greater risk for complications from COVID-19," she said.
The researchers called for vaccine developers to recognize the importance of including pregnant and breastfeeding women in clinical trials. (Pfizer began its first study in this group in February 2021.)
"The potential for rational vaccine design to drive improved outcomes for mothers and infants is limitless, but developers must realize that pregnancy is a distinct immunological state, where two lives can be saved simultaneously with a powerful vaccine," co-senior author Galit Alter, PhD, said in the release. "We look forward to studying all vaccine platforms in pregnancy as they become available."