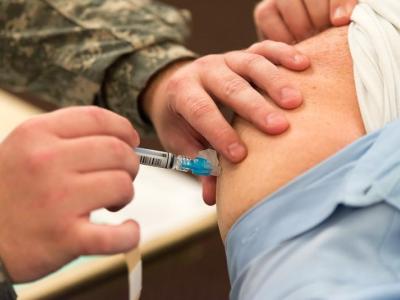
Monkeypox vaccine 79% effective, according to Israeli preprint study
According to a new non-peer reviewed study out of Israel, the Jynneos vaccine is 79% effective against monkeypox infection.
The study was based on patients eligible for monkeypox vaccine seen in the Clalit Health Services system between Jul 31 and Sep 12, 2022. Of 1,970 subjects eligible for the study, 873 (44%) were vaccinated with Jynneos and completed at least 25 days of follow-up.
Fifteen unvaccinated subjects and three vaccinated participants contracted monkeypox during the study, with vaccine effectiveness estimated at 79% (95% CI [confidence interval], 24% to 94%).
In related news, the US Centers for Disease Control and Prevention has reported 274 more monkeypox cases, raising the total to 24,846. So far, 3,233 have been treated with the antiviral Tpoxx, and 684,980 vaccine doses have been administered in the United States.
Siga, the company behind Tpoxx, announced $16 million of international procurement orders, including $10 million from Europe and $6 million from Asia. The company said it expects to deliver on the orders before the end of the year.
Because the virus has been circulating in the United States for 4 months, more and more monkeypox patients are technically immune after infection but may have lingering scars, anxieties, and fears, according to NBC News. Monkeypox scarring can be disfiguring, especially in the genitals, and some men are complaining of lingering proctitis (inflammation of the lining of the rectum).
Sep 26 Research Square Israeli preprint study
Sep 26 CDC update
Sep 26 CDC Tpoxx update
Sep 26 CDC Jynneos update
Sep 26 Siga press release
Sep 25 NBC News story
Department of Defense funds research of monoclonal antibodies for plague
German pharmaceutical company Evotec SE announced last week that its US subsidiary (Just – Evotec Biologics) has received nearly $5o million in funding from the US Department of Defense (DOD) to develop a monoclonal antibody (mAB)-based drug against bubonic plague.
Under the $49.9 million contract, Just – Evotec Biologics, of Seattle, will develop a mAB-based drug product prototype from sequence discovery through completion of phase 1, first-in-human clinical trials, with the aim of producing an accelerated supply of safe and effective mAB medical countermeasures against plague, which is caused by the bacterium Yersinia pestis.
Plague is one of the targets of the DOD's Accelerated Antibodies Program.
"We are delighted to support the DOD with this work of strategic national importance, and which we feel represents a clear validation of both the efficiency and speed our leading science, technology and expertise offers our partners," Craig Johnstone, PhD, Evotec chief operating officer, said in a press release.
Sep 20 Evotec press release
High-path avian flu strikes more poultry in 9 states
Nine states reported more highly pathogenic avian flu in poultry, which mostly involved backyard poultry, though four states had more outbreaks in commercial flocks, one of them a large layer farm in Colorado, according to the latest updates from the US Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS).
In Colorado, the outbreak struck an egg producer in Weld County that houses 1.15 million birds. Other states reporting events at commercial farms include Idaho, where the virus hit a game bird producer in Gooding County that has 9,000 birds. Minnesota reported outbreaks at two more turkey farms, one in Otter Tail County and one in Roseau County, that taken together led to the loss of 118,000 birds. South Dakota also reported an outbreak at a turkey farm, which is located in McPherson County and has 15,000 birds.
Seven states reported more outbreaks in backyard birds: Delaware, New Hampshire, Maryland, Minnesota, North Dakota, South Dakota, and Texas.
Since the H5N1 outbreaks in poultry began in early February, backyard and commercial poultry operators in the United States have lost 46.4 million poultry across 40 states. A small but steady stream of activity in poultry occurred over the summer but has gained steam over the past month, especially in the Midwest and West.
USDA APHIS poultry outbreak updates
A number of European countries have also reported new H5N1 outbreaks, including a new event in Italy that involves backyard birds in Veneto region, according to a notification from the World Organization for Animal Health (WOAH). The outbreak—the country's first since April—began on Sep 21 in Treviso province, killing 10 of 30 birds.
Sep 26 WOAH update