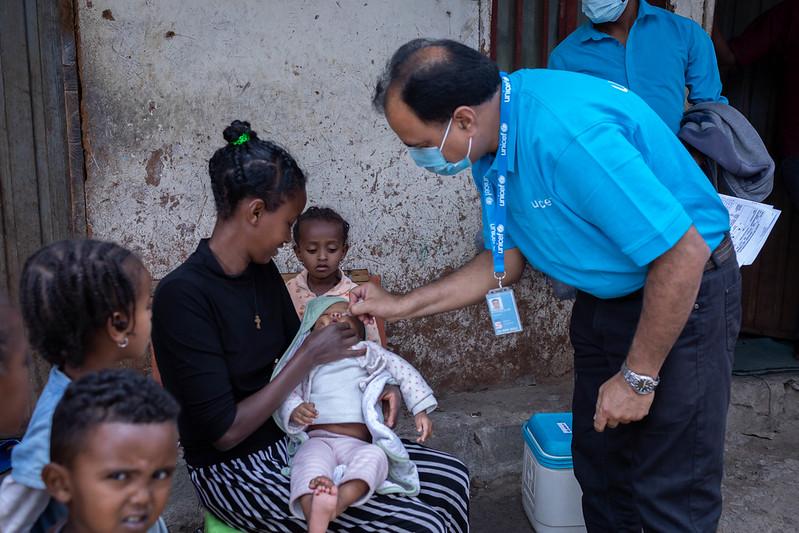
The World Health Organization (WHO) recently prequalified the novel oral poliovirus vaccine type 2 (nOPV2), the first time the group has ever prequalified a vaccine that is being used under the emergency use listing, the Global Polio Eradication Initiative (GPEI) announced yesterday.
Rollout of the new vaccine began in March 2021, and nearly 1 billion doses have already been administered across 35 countries. WHO prequalification, though, paves the way for more countries to receive the vaccine, which has played a key role in stemming outbreaks involving type 2 variant poliovirus (cVDPV2).
In GPEI's statement, WHO Director-General Tedros Adhanom Ghebreyesus, PhD, called the prequalification a historic public health milestone. "Novel oral polio vaccine type 2 has blazed a trail for other new vaccines that address critical health emergencies, and its use demonstrates the utility of the EUL mechanism in helping to rapidly get new products to where they’re needed most."
GPEI added that WHO prequalification streamlines regulatory approval in countries that need it.
Consortium-developed vaccine
The vaccine's maker is Bio Farma Indonesia, and regulators in Indonesia have fully licensed it. It was developed by an international consortium that included the Bill and Melinda Gates Foundation, the United Kingdom's National Institute for Biological Standards and Controls (NIBSC), the US Centers for Disease Control and Prevention, the US Food and Drug Administration, PATH, and the University of California at San Francisco.
Oral polio vaccine (OPV) is widely used in campaigns in lower-resource countries, because it is easy to store and transport and easy to administer. However, the attenuated virus in the oral vaccine can mutate and become virulent again, especially in settings where uptake is low.
GPEI said nOPV2 is as safe and effective as its predecessor, monovalent type 2 oral polio vaccine (mOPV2), but is more genetically stable. Estimates after 3 years of clinical use suggest nOPV2 is 80% less likely to seed new variant polio outbreaks.
Nigeria was one of the countries hardest hit by cVDPV2 outbreaks and has played a key role in nOPV2 rollout, GPEI said. Over the past 3 years, use of the vaccine reduced Nigerian cases by 85%. Globally, 325 cases were reported in 2023, down sharply from 689 cases in 2022.