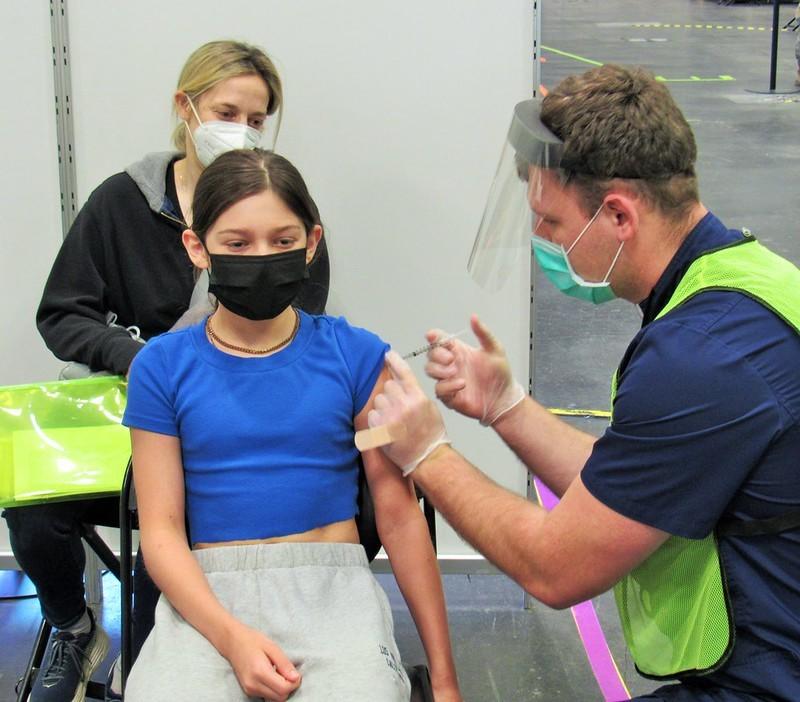
The Biden administration has procured enough Pfizer-BioNTech COVID-19 vaccines to inoculate the 28 million American children ages 5 to 11 who are set to become eligible for vaccination as soon as this week.
Speaking today during a White House press briefing, Jeff Zients, the administration's COVID-19 response coordinator, said the formal pediatric vaccination program will officially begin on Nov 8, pending the expected recommendation from the Centers for Disease Control and Prevention (CDC).
"As we await CDC's decision, we are not waiting on operations or logistics," Zients said.
The CDC's ACIP (Advisory Committee on Immunization Practices) is meeting tomorrow to discuss the emergency use authorization application for the Pfizer vaccine in children ages 5 to 11, which was granted late last week by the Food and Drug Administration (FDA). Zients told National Public Radio this weekend that parents will be able to find pediatric vaccinations on vaccine.gov, and vaccines will be available in 20,000 locations across the country.
In other vaccine news, Zients said that by the end of today 20 million Americans will have received booster doses of one of the three COVID-19 vaccines approved for use in adults.
The CDC COVID Data Tracker shows 58% of Americans are fully vaccinated against COVID-19, 66.7% have received at least one dose of vaccine, and 9.7% of fully vaccinated Americans have received a booster dose.
Moderna application delayed
The Pfizer-BioNTech vaccine may be the only vaccine available to children in the United States, at least until January, as Moderna has been notified by the FDA that a full analysis of its application for vaccine use in 12- to 17-year-olds needs more time for review.
Yesterday Moderna said the FDA notified the company over the weekend, and said the agency requires additional time to evaluate due to "recent international analyses of the risk of myocarditis after vaccination," according to a company press release.
"The FDA notified Moderna that this review may not be completed before January 2022," the company said. "The safety of vaccine recipients is of paramount importance to Moderna. The Company is fully committed to working closely with the FDA to support their review and is grateful to the FDA for their diligence."
Moderna said to date 1.5 million adolescents have received the Moderna COVID-19 vaccine, and the observed rate of myocarditis reports in those less than 18 years does not suggest an increased risk of myocarditis in this population.
The move was likely prompted by an unpublished study by Sweden's Public Health Agency, which showed a slight increased risk of myocarditis. Moderna said it has not had access to those data.
The United States reported 17,599 new COVID-19 cases yesterday, and 164 deaths, according to the Johns Hopkins COVID-19 tracker.
Finally, today marks the first day of New York City's COVID-19 vaccine mandate for municipal workers, the New York Times reports. Roughly 91% of city workers have gotten vaccinated, Mayor Bill DeBlasio said, but thousands who refused are expected to be told to stay home.