Researchers at Dartmouth Hitchcock Medical Center have developed a way to detect Lyme disease more rapidly and reliably than current testing methods. The team presented the new molecular test late last week at the Association for Molecular Pathology 2025 Annual Meeting & Expo.
The project originated with a patient whose antibody tests showed evidence of previous, but not current, Lyme infection (existing serologic tests cannot distinguish between active and past infection).
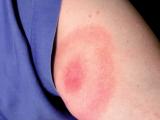
When the patient's symptoms (skin hardening, inflammation, and joint immobility) improved with doxycycline, an antibiotic often used to treat Lyme disease, her care team asked researchers to develop a molecular test to confirm the presence of Borrelia burgdorferi, the bacterium that causes most Lyme disease cases in the United States.
Ability to detect as few as 5 bacterial cells
The team developed three droplet digital polymerase chain reaction (ddPCR) tests to detect different types of Borrelia bacteria: one identifies all Borrelia species; one finds all Borrelia species responsible for Lyme disease; and one targets B burgdorferi. The new assays detected as few as five to 10 bacterial cells, which the researchers describe as "excellent accuracy."
The B burgdorferi test had 91% sensitivity in detecting the bacteria. "Using the ddPCR assay, we successfully detected B. burgdorferi DNA in this patient's skin biopsy," explains lead researcher Guohong (Grace) Huang, PhD, in the press release. "This finding was further confirmed by DNA sequencing, supporting the diagnosis of chronic Lyme disease."
"Antibody levels may remain elevated even after successful treatment. This is another clinical scenario where the ddPCR assay offers a clear advantage by detecting active bacterial presence rather than relying on indirect immune markers," adds Huang.