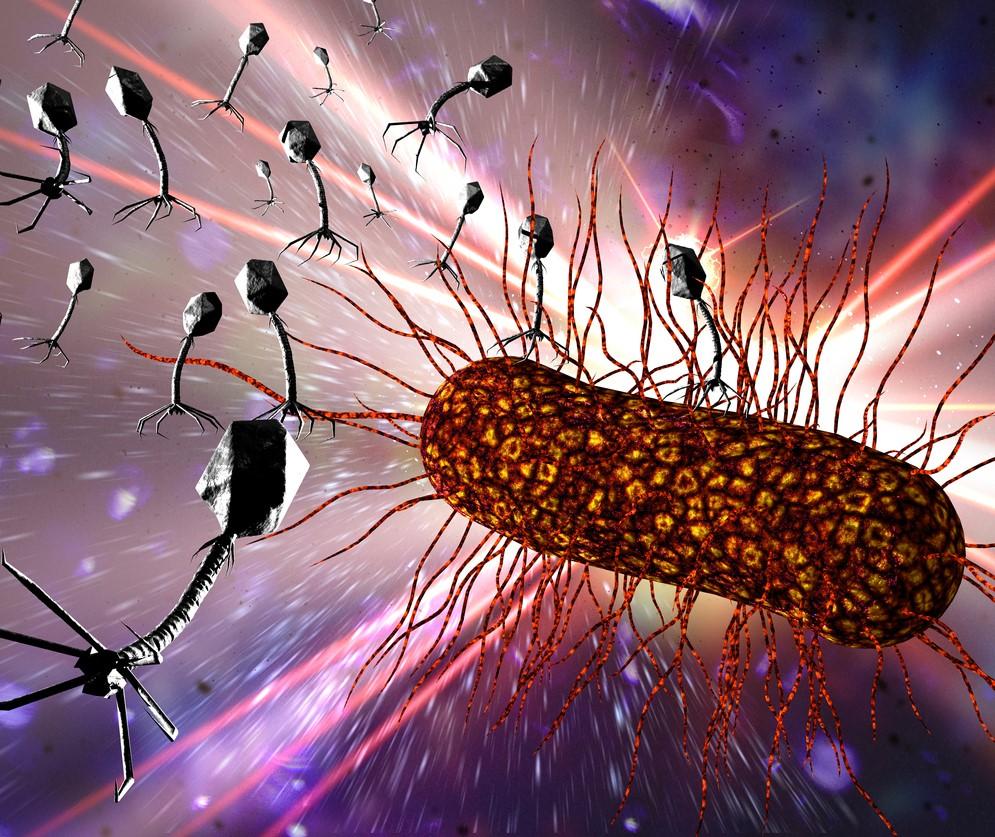
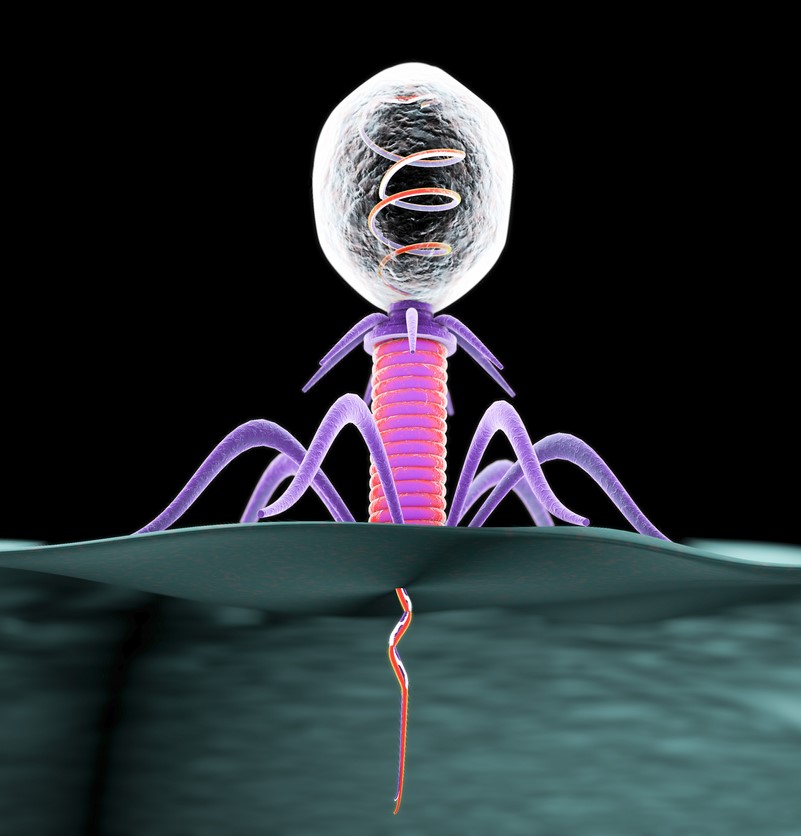
Bacteriophages are everywhere.
Considered the most abundant organisms on the face of the earth, these microscopic viruses that selectively target and kill bacteria can be found wherever bacteria exist—in soil, inside plants and animals, and in oceans, streams, and sewage water.
Discovered more than a century ago, phages were at one point viewed as a potential weapon against bacterial infections. Then came the antibiotic era. Now, with antibiotic resistance spreading and the threat of a post-antibiotic future looming, scientists are again turning to the ubiquitous bacteria-killing viruses for answers.
Phage therapy in the limelight
In recent years, the potential for phages to treat patients with life-threatening antibiotic-resistant infections has been highlighted by several high-profile emergency use cases.
In 2016, there was the case of Tom Patterson, PhD, a college professor with a severe, multidrug-resistant Acinetobacter baumannii infection who recovered after an intravenous (IV) infusion of a cocktail of phages. Patterson was the first US patient to successfully undergo IV bacteriophage therapy.
 In 2018, phage therapy was in the news again when Isabelle Carnell-Holdaway, a 15-year-old cystic fibrosis (CF) patient struggling with a multidrug-resistant Mycobacterium abscessus infection following a double-lung transplant, was successfully treated with a cocktail of natural and genetically modified phages.
In 2018, phage therapy was in the news again when Isabelle Carnell-Holdaway, a 15-year-old cystic fibrosis (CF) patient struggling with a multidrug-resistant Mycobacterium abscessus infection following a double-lung transplant, was successfully treated with a cocktail of natural and genetically modified phages.
These are just two of the handful of recent cases where clinicians, facing severe and life-threatening infections that were no longer responsive to antibiotics, have successfully resorted to phage therapy. There is increasing recognition in the medical community that phages could an important tool as the threat of antibiotic resistance grows.
"We've really started to see the infectious disease community embrace the idea of phage therapy," says Steffanie Strathdee, PhD, co-director of the Center for Innovative Phage Applications and Therapeutics (IPATH) at the University of California, San Diego. "There's greater awareness, a sense of urgency, and a growing consensus that phage therapy is one of the most important alternatives or adjuncts to antibiotics that's out there."
A laborious process
But the process of finding phages that match the strain of infecting bacteria, getting them prepared for use in a patient, and getting regulatory approval for emergency or compassionate use in that patient is time-consuming and laborious. There's also the question of whether a treatment that's so specific—in most cases, phages need to match the particular strain of bacteria causing an infection in a patient in order to work—can be scaled up for wider clinical use.
The high specificity that phages have for their bacterial hosts is what University of Pittsburgh phage researcher Graham Hatfull, PhD, calls a "double-edged sword."
"On the one hand, you have these precision missiles that can go and take out a particular pathogen without disturbing the rest of the body and the rest of the microbiome," says Hatfull, who was part of the team that identified and developed the phage cocktail for Carnell-Holdaway.
"But on the other hand, if it's so specific that you have trouble accessing a wide range of bacterial isolates of any particular pathogen, then that makes your task really difficult."
It's a problem that several researchers and small biotechnology companies are trying to solve as they look to make phage therapy more broadly applicable.
An 'ever-expanding' phage library
Adaptive Phage Therapeutics (APT) is one of those companies. Based in Maryland, APT own exclusive rights to a bacteriophage library created at the Biological Defense Research Directorate of the US Navy. The phage library was conceived by former National Institutes of Health (NIH) scientist Carl Merril, MD, who would go on to start APT with his son Greg. The library contributed phages from sewage water to the therapy that saved Patterson's life.
APT is now using this ever-expanding phage library, which it calls PhageBank, to provide phages for other emergency use cases under the Food and Drug Administration's (FDA's) expanded access pathway. PhageBank is paired with a phage susceptibility assay developed by the Mayo Clinic that matches the specific bacterial strain infecting a patient to a phage, or phages, that are lytic—which means they target the selected bacteria, inject their DNA into the bacterial cell to reproduce more phages, then destroy the bacteria from within.
"So, for example, if a patient has a chronic prosthetic joint infection, they show up at the hospital, and the treating physician would send that culture to the lab," says APT CEO Greg Merril. "In our case, we have a partnership with the Mayo Clinic, and the vision is that the Mayo Clinic laboratories would test the bacterial isolate against this phage collection to identify the specific phage that's lytic against that patient's bacteria." 
PhageBank has been used successfully in several compassionate use cases in the United States, Europe, and Israel. But the company is looking beyond individual cases and has initiated clinical trials of this process in patients with chronic prosthetic joint infections, chronic urinary tract infections, and chronic wound infections with osteomyelitis. The aim, ultimately, is to have the FDA approve the process for patients who have infections that are not responding to antibiotic treatment.
"We see phage coming in to use for recalcitrant, difficult-to-treat infections, where you're still using standard-of-care antibiotics and you're adding the phage in as an additional therapy," says Greg Merril. "That's one of the reasons why we've focused on clinical indications like chronic prosthetic joint infections, where standard of care is really just suboptimal for these patients."
In addition to lytic phages, Merril says APT is also interested in phages that can eradicate biofilms, which are complex, multicellular bacterial clusters that tend to form on artificial joints and devices like urinary catheters.
Merril explains that the process of continuously testing patient isolates for susceptibility to phages, and finding the phages that bacteria have developed resistance to, will help the company keep expanding its library. Bacterial isolates that aren't covered by phages in the library are used as targets for new phage discovery.
"The only way we could see of creating a drug product that is able to avoid this cycle of obsolescence is to incorporate surveillance of the bacteria as it evolves resistance and continuing to enrich the PhageBank with new phage that the bacteria aren't resistant to," he says.
Killing bacteria, driving evolutionary tradeoffs
In New Haven, Connecticut, Benjamin Chan, PhD, is among a group of Yale University researchers and clinicians looking at the potential for using phage therapy in CF patients, whose condition leaves them susceptible to chronic pulmonary infections.
"We're doing work in CF because that population is exposed a lot to antibiotic-resistant infections," he says. "Because of the disease and the predisposition for bacterial infection, they get a lot of antibiotics, and so we see a lot of antibiotic-resistant bacteria in that group."
Specifically, Chan and his colleagues are looking at how phages can help with infections caused by Pseudomonas aeruginosa, a multidrug-resistant pathogen that is the most common cause of chronic lung infections in CF patients. In a phase 1/2 clinical trial that started enrolling patients earlier this year, the investigators are evaluating the safety and efficacy of nebulized phage therapy using a phage cocktail, YPT-01, that targets P aeruginosa. The primary outcome measure is reduction of the bacterial load as seen in sputum culture.
 YPT-01, which has been used in CF patients on an emergency use basis, not only targets and kills P aeruginosa, it can also drive evolutionary tradeoffs by targeting bacterial mechanisms that make the pathogen more virulent or more resistant to antibiotics. What Chan and his colleagues have found is that, in some cases, the bacteria will alter these mechanisms to avoid the phage. But they can fight only one enemy at a time.
YPT-01, which has been used in CF patients on an emergency use basis, not only targets and kills P aeruginosa, it can also drive evolutionary tradeoffs by targeting bacterial mechanisms that make the pathogen more virulent or more resistant to antibiotics. What Chan and his colleagues have found is that, in some cases, the bacteria will alter these mechanisms to avoid the phage. But they can fight only one enemy at a time.
"It's beneficial when bacteriophages are able to kill off the bacteria that's causing an infection, of course, but when bacteria evolve resistance to the phages—as they'll evolve resistance to any intervention—then it comes with some clinical benefit," Chan explains. "In our case, our hope is that we can get phages that can drive a tradeoff to improve antibiotic sensitivity, or attenuate virulence in some cases. That's a really nice feature of phages."
Building the infrastructure
Strathdee, who's married to Tom Patterson and co-wrote a book about his experience called The Perfect Predator, says more clinical trials are needed to advance phage therapy beyond compassionate use cases. But they're not happening fast enough, she says.
"Look at how quickly we were able to get COVID vaccines and therapeutics rolled out," she says. "We need to ensure that same sense of urgency reaches Congress and policymakers and funders of other kinds to ramp this up."
The infrastructure that would enable wider use of phage therapy, even if it remains mainly under emergency use conditions, also needs to be developed, she says. Between June 2018 and April 2019, IPATH received 785 requests for bacteriophage therapy from patients. IPATH works with a network of collaborators around the world to hunt phages and match them to patients, but many of those collaborators are also swamped with requests.
And while phages may be everywhere, that doesn't mean they are easy to cultivate.
"There's definitely a lot of work that goes into finding, and characterizing, and making sure that we're doing this safely and effectively," says Chan.
Finding the phages is only part of the process. The phages then have to be amplified and purified to remove the toxic material—like the remnants of the bacterial cell wall—that's left behind in the lytic process. This is particularly important for intravenous administration of phages.
"If you're sticking something into somebody's veins, you really would like to have some confidence that's it's been highly purified," Hatfull says.
While APT has developed its own manufacturing plant to grow and purify PhageBank phages, in many cases, researchers with phage collections don't have the ability to prepare their phages for clinical use. Strathdee recounted a recent case in Europe involving phages that were identified in Israel, sent to Brussels to be expanded and purified, then sent to a patient in Germany. That's not a sustainable situation in the long run.
"When a patient is dying, every minute counts," she says. "So what we really need is not just the phage library that's ever-expanding, but we need partners, GMP [Good Manufacturing Practices] facilities, and at least one on every continent so that they'll be able to get the phage ready and prepared on time for the patient."
Once that infrastructure is in place, most phage researchers and advocates foresee phage therapy not as a replacement for antibiotics, but as an adjunct that works synergistically with antibiotics to defeat multidrug-resistant infections and potentially reduce the amount of antibiotics that are needed. That could help lower some of the selection pressure that drives antibiotic resistance.
"We don't imagine phage is ever going to replace antibiotics, but they are going to be offered in tandem," Strathdee says. "That's the other exciting area—if we could anticipate which phage and which antibiotics go well together, then we could use fewer antibiotics and potentiate the impact."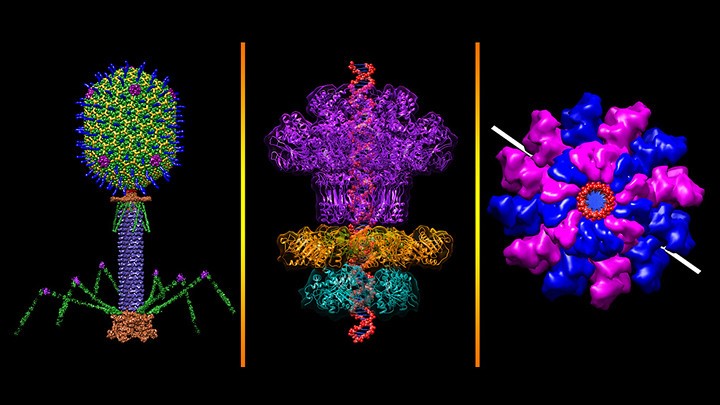
Hatfull says that while the path forward is unclear right now, a future where phages are a boutique, personalized intervention for difficult-to-treat or life-threatening infections seems achievable.
"I would like to think that there are at least some opportunities where phages likely, or could, come into pretty common use to control a particular infection, probably alongside antibiotics," he says. "It should be achievable, and I hope it will be affordable, but I think that is still something of a moving target that isn't completely pinned down."
A tool for cholera control
In Boston, a company called PhagePro is exploring a different path, one that would harness the bacteria-killing potential of phages to benefit some of the most impoverished parts of the world. Company co-founder and CEO Minmin Yen, PhD, MPH, calls it a "phages for all" approach.
Yen and her colleagues are in the pre-clinical stages of developing ProphaLytic-VC, an orally dosed bacteriophage cocktail that would target endemic strains of cholera, an acute diarrheal disease that the World Health Organization estimates kills anywhere from 21,000 to 143,000 people a year, mostly in places with poor access to clean water and sanitation. They envision the product being used, as its name suggests, preventively, by close contacts of people with cholera infections.
Yen says a fast-acting prophylactic could have tremendous benefits for reducing household transmission, which plays a significant role in cholera outbreaks. Household members frequently start showing cholera symptoms within 2 days after the initial patient becomes sick. Yen envisions ProphaLytic-VC as complementing oral cholera vaccines and water and sanitation interventions.
"As opposed to a vaccine—the other very common preventative that relies on the immune system but takes several weeks in order to provide protection—phages are much more direct and targeted in their approach," she says. "So it would provide immediate protection and hopefully would be able to disrupt transmission in that short amount of time."
The cocktail includes three virulent phages that Yen and PhagePro co-founder Andrew Camilli, PhD, tested against Vibrio cholerae—the bacterium that causes cholera—in mice and rabbits. In a paper published in Nature Communications in 2017, they showed that oral administration of the phages 24 hours before V cholerae infection reduced colonization of the intestinal tract and prevented cholera-like diarrhea.
While the specificity of bacteriophages can be a barrier to creating this type of off-the-shelf treatment for many bacterial infections, Yen says that cholera is less of a challenge because although there are many V cholerae strains in the environment, only a few strains are responsible for epidemics.
 "If we're thinking about a cocktail of phages that can cover cholera all over the world, then cholera being clonal in nature helps with achieving that broad host coverage, because there aren't that many strains that can cause that level of disease," she says.
"If we're thinking about a cocktail of phages that can cover cholera all over the world, then cholera being clonal in nature helps with achieving that broad host coverage, because there aren't that many strains that can cause that level of disease," she says.
PhagePro recently received a $3 million grant from the National Institutes of Health to further its work on ProphaLytic-VC. Yen says the money will go toward getting the product into first-in-human trials and turning it from a liquid into a pill form. She and her colleagues also want to determine whether it could be co-administered with the cholera vaccine.
Yen says she hopes the work on ProphaLytic-VC, if it proves effective, could help lay the groundwork for phages to be a significant global health tool against other enteric bacterial diseases that ravage poorer parts of the world.
"I think that phages have a huge potential when it comes to reducing diseases, specifically when it comes to the burden of diarrheal diseases in public health," she says.
APT's Greg Merril is also thinking big. He envisions PhageBank being used for other acute, multidrug-resistant infections like hospital-acquired pneumonia.
"There's going to be a really rapid expansion of the use of phage to treat antimicrobial resistant [AMR] infections," he says. "I think there is an opportunity in the next 10 to 15 years to eradicate AMR as the big potential threat that it currently is."