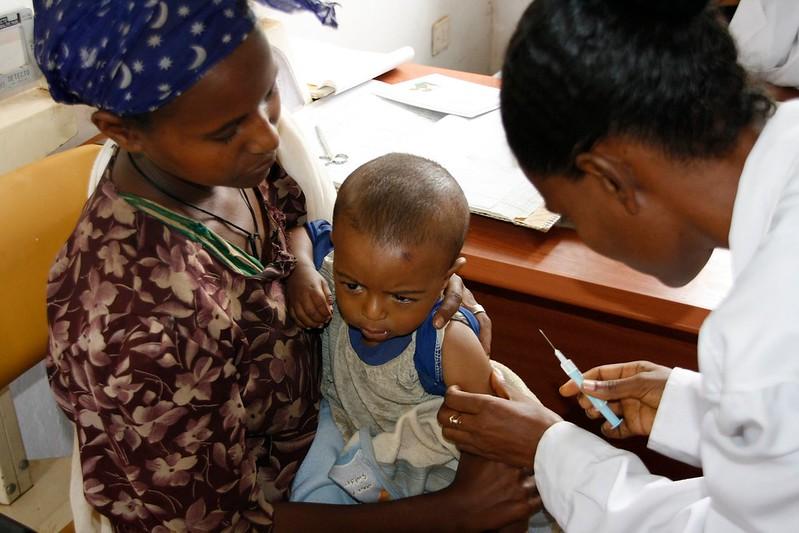
Against the backdrop of unprecedented demand for a new malaria vaccine, the World Health Organization (WHO) today recommended a second malaria vaccine called R21/Matrix-M, which is expected to boost supply.
The recommendation came following an extensive safety and efficacy review by the WHO's Strategic Advisory Group of Experts on Immunization (SAGE) and its Malaria Policy Advisory Group. The vaccine was developed by the University of Oxford and is licensed and made by the Serum Institute of India. The company has said it has the capacity to make 200 million doses each year.
Health officials estimate that half a million children in Africa die each year from malaria, and the arrival of vaccines has been eagerly anticipated. So far, at least 28 African countries have said they will include the malaria vaccine in their routine immunization programs. In 2021, the WHO recommended the first malaria vaccine, RTS,S/AS01.
Faster path to a malaria-free future
WHO advisors based their recommendation on ongoing clinical trials, which suggest R21 has high efficacy when given ahead of high malaria transmission season, similar to that for seasonal use of RTS,S. The vaccine had good efficacy after three doses, with levels maintained with a fourth dose given 1 year after the third dose.
At $2 to $4 dollars per dose, the vaccine's cost-effectiveness is thought to be comparable to other malaria interventions and childhood immunizations. The vaccines have not been tested in head-to-head trials, and the WHO said use of either vaccine should depend on vaccine program requirements, supply, and affordability.
In a WHO statement, WHO Director-General Tedros Adhanom Ghebreyesus, PhD, said that as a former malaria researcher, he dreamed of a day when the world had a safe effective vaccine. "Now we have two," he said. "Demand for the RTS,S vaccine far exceeds supply, so this second vaccine is a vital additional tool to protect more children faster, and to bring us closer to our vision of a malaria-free future."
Matshidiso Moeti, MBBS, who directs the WHO's African regional office, said the vaccine has the potential to fill the demand-and-supply gap. "Delivered to scale and rolled out widely, the two vaccines can help bolster malaria prevention and control efforts and save hundreds of thousands of young lives in Africa from this deadly disease."
As the next step, the WHO will complete the prequalification process, which would pave the way for countries and groups to buy the vaccine for a broader rollout. The RTS,S vaccine will launch in some African countries in 2024, and R21 is expected top become available in the middle of 2024.
Health groups welcome second vaccine
In an e-mail press release today, PATH, a global nonprofit group focused on health equity, welcomed the recommendation for a second malaria vaccine. John Bawa, director of malaria vaccine implementation at PATH, said the availability of a second vaccine will speed the pace of vaccine introduction, save lives, and should help to reduce prices.
He estimated that with the support of Gavi, the Vaccine Alliance, most African countries will pay about 20 cents per vaccine dose.
Gavi also welcomed today's recommendation. David Marlow, the group's chief executive officer, said the recommendation is another step forward in creating a malaria-free life for every child. "This vaccine, along with the existing RTS,S/AS01e vaccine, will be an effective complement to existing malaria interventions. Once it receives WHO prequalification, it will play a key role in meeting the high demand we are seeing in endemic countries," he said.
Other SAGE recommendations cover dengue, malaria, and COVID vaccines
In other developments, SAGE made recommendations regarding three other vaccines. It recommended a new vaccine against dengue infection. Made by Takeda, Qdenga is recommended for children ages 6 to 16 years old living in areas where the disease is endemic.
The group also recommended a new vaccine against meningitis for countries in Africa's meningitis belt. Called Men5CV, the vaccine targets five serogroups (A, C, Y, W, and X). The vaccine would be given as a single dose between 9 and 18 months, with a catch-up campaign in high-risk countries in those ages 1 to 19 years old.
And finally, the group recommended a single-dose strategy for primary immunization with most COVID vaccines, given that most people have had at least one prior infection. SAGE also said in settings where the updated monovalent XBB vaccine isn't available, any WHO-listed COVID vaccine can be used, because they continue to help protect against severe disease in high-risk groups.