- The US Centers for Disease Control and Prevention (CDC) yesterday reported 32 new cases in a Salmonella Typhimurium outbreak linked to cucumbers imported from Mexico, raising the total to 100 people from 23 states. Seven more people were hospitalized for their infections, raising the total to 25. No deaths have been reported. The CDC and states have identified seven subclusters at three assisted-living facilities, three school districts, and one restaurant. The outbreak was first announced in late November. The trace-back investigation led to a common grower in Mexico, which resulted in a recall of the cucumbers.
- Gavi, the Vaccine Alliance, today announced that it has wrapped up work on an agreement to facilitate donations of 305,000 doses of Bavarian Nordic mpox vaccine on behalf of the United States to affected African countries. The first delivery of 11,200 doses is expected to arrive in Nigeria today. In late September, the United States announced a plan to donate up to 1 million doses to support the public health emergency for mpox, which launched discussions to donate the first 305,000 doses through Gavi. About 6 million mpox vaccine doses have been pledged by different countries and groups, with delivery expected in 2024. Some doses have already been allocated to nine of Africa's hardest-hit countries. Gavi said it is still in discussions with the United States about the remaining pledged doses.
- In its weekly update, the Global Polio Eradication Initiative (GPEI) reported more polio cases in three countries. Pakistan reported 4 more wild poliovirus type 1 (WPV1) cases in Khyber Pakhtunkhwa and Sindh, all in patients with November paralysis onsets, raising the country's total for the year to 63. In Africa, two countries reported more circulating vaccine-derived poliovirus type 2 (cVDPV2) cases: Chad with 4 and Nigeria with 3. The countries have reported 26 and 89 cases this year, respectively.
Quick takes: More cucumber-linked Salmonella, mpox vaccine for Africa, polio in 3 nations
CWD confirmed in captive Idaho elk for first time, expands its range in Washington state

Chronic wasting disease (CWD) has been detected in a captive elk for the first time in Idaho and in a newly affected hunt area in neighboring Washington.
Elk was imported from Canada
The Idaho State Department of Agriculture (ISDA) yesterday said a domestic bull elk tested positive for CWD in Madison County. It had died earlier, and tissue samples were submitted for routine testing. The US Department of Agriculture's National Veterinary Services Laboratory confirmed the findings.
The infected bull was among a group of elk transported to the Idaho ranch in March 2023 from a facility in Alberta, Canada. The elk facility had been approved to import the herd. Shortly after the shipment arrived in Idaho, the ranch in Alberta confirmed a CWD-positive elk.
"Once ISDA was notified of the CWD-positive elk from the Canadian ranch, the shipment that arrived in Idaho was placed under a protective quarantine to restrict further movement of the CWD-exposed animals," the agency said. "All remaining elk that arrived in the 2023 shipment are alive and will remain under state-issued quarantine."
CWD, an always-fatal prion disease that affects member of deer family, was first detected in wild deer in Idaho in 2021, and the following year in wild elk.
Washington CWD total reaches 6
The Washington Department of Fish and Wildlife (WDFW), meanwhile, yesterday confirmed four new CWD cases in Eastern Region 1, bringing the total CWD cases in Washington to six.
All four of the recent cases were hunter-harvested white-tailed bucks. Three of the deer were harvested within 5 miles of the first two positive cases in Spokane County in game management unit (GMU) 124. The fourth was several miles north, near Davis Lake in Pend Oreille County in GMU 117, the first detection in that hunt unit.
We could find additional positive cases.
"There are still several samples awaiting testing at the lab from the areas where these recent cases were confirmed," said Donny Martorello, PhD, chief of the WDFW's Wildlife Science Division. "So, there is the potential that we could find additional positive cases."
Rwanda's Marburg outbreak declared over
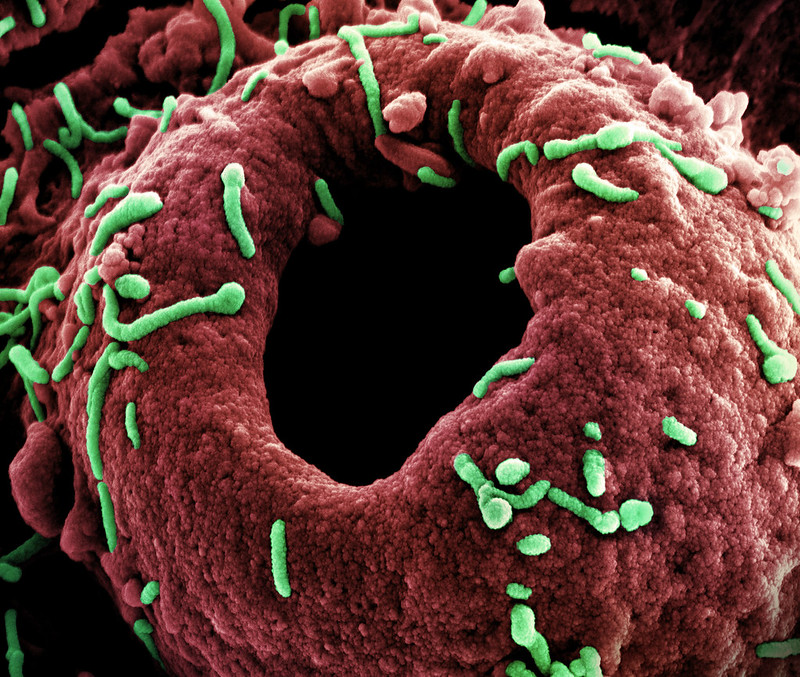
The Rwandan government today declared the end of the country's Marburg virus outbreak, after passing 42 days with no new cases since the last patient was discharged from the hospital. The time period represents two virus incubation periods.
Declared on September 27, the outbreak was Rwanda's first involving the virus. The event resulted in 66 cases, 15 of them fatal. Most of the infections were reported in healthcare workers who cared for their colleagues and other patients at two hospitals in Kigali.
The case-fatality rate was about 23%, which is at the low end of the 24% to 88% range.
WHO lauds response efforts
In a statement the World Health Organization (WHO) today praised the comprehensive response of Rwanda and its partners. Brian Chirombo, MD, MPH, the WHO's representative in Rwanda, said, "The robust response by Rwanda shows how committed leadership, concerted efforts by partners, and a strong health system are crucial in addressing public health emergencies, saving and protecting lives, as well as safeguarding the health of individuals and communities."
The robust response by Rwanda shows how committed leadership, concerted efforts by partners, and a strong health system are crucial in addressing public health emergencies, saving and protecting lives, as well as safeguarding the health of individuals and communities.
The index patient is thought to have contracted the virus in a mining-area cave where fruit bats, known to carry the virus, were present. Genetic sequencing found that the virus is similar to Marburg viruses that had been reported in that region of Africa before.
As part of the outbreak response, the country deployed an investigational Marburg vaccine from the Sabin Vaccine Institute, which was used to immunize healthcare workers in conjunction with a clinical trial. Rwanda's scientists also launched the world's first clinical trials for Marburg treatments in conjunction with their WHO partners.










