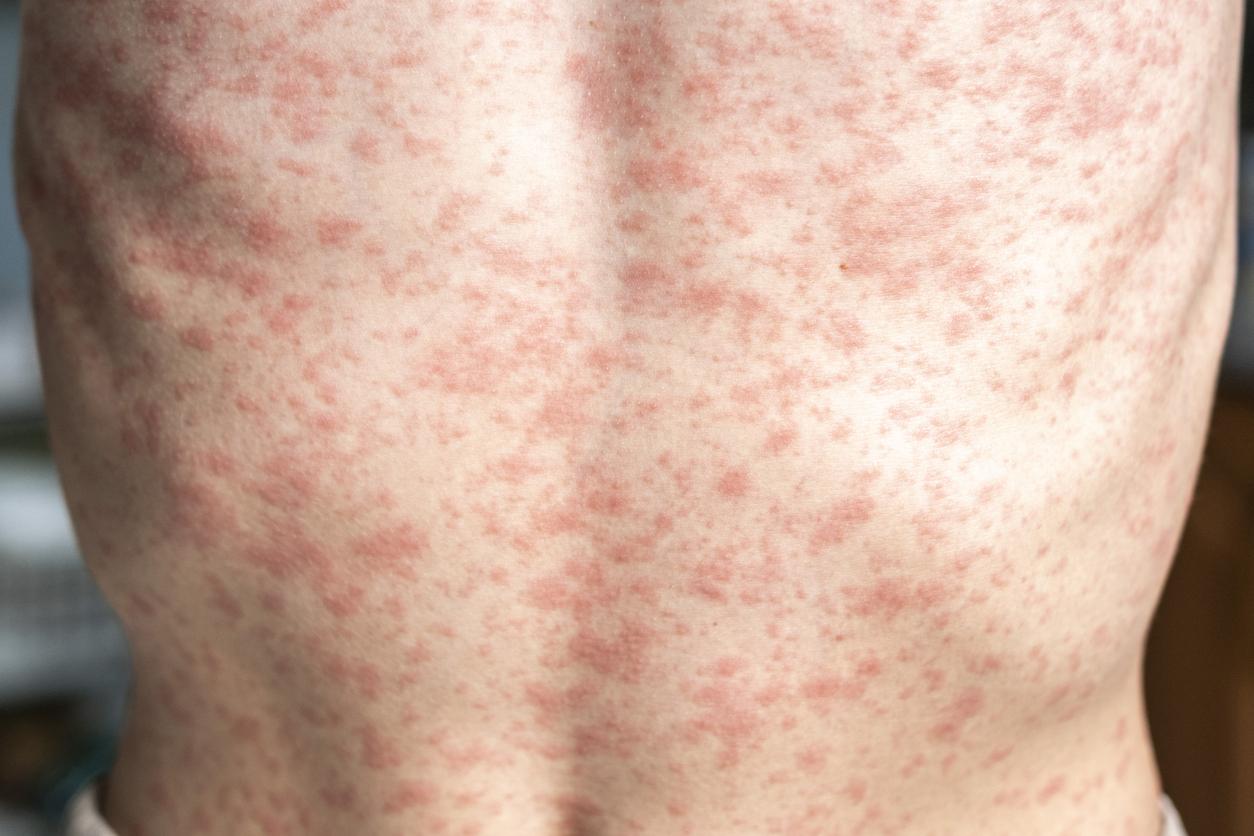
Danish drugmaker Bavarian Nordic announced today that the US government has placed a $144 million order for a freeze-dried version of its mpox vaccine, which can also be used to prevent smallpox.
The freeze-dried formulation of the Jynneos vaccine was approved by the US Food and Drug Administration (FDA) in March. It will be easier to store and transport, and has a longer shelf life, than the liquid-frozen formulation that Bavarian Nordic has been supplying to the US government since 2010. The company ramped up production of Jynneos in response to the mpox outbreak that began in 2022.
Jynneos was first approved by the FDA in 2019 and is one of two FDA-approved vaccines for the prevention of smallpox and mpox.
The options exercised under Bavarian Nordic's existing contract with the Biomedical Advanced Research and Development Authority (BARDA), which was awarded in 2017, are valued at $143.6 million and will support manufacturing and supply of freeze-dried Jynneos starting in 2026.
"We applaud the U.S. government’s steadfast commitment to improving national health security through the exercise of these options," Bavarian Nordic President and CEO Paul Chaplin, PhD, said in a company press release. "The freeze-dried vaccine, with its improved shelf life, provides a significant contribution to securing the long-term availability of countermeasures to protect U.S citizens against life-threatening diseases."
.jpg)
.jpg)










